TEAMFunctional Group Transformation Team

Various useful properties and functions of materials are appeared from their molecular frames and functional groups of which the materials consist. This group aims to contribute following three fields of catalysis chemistry related with transformation of molecular frames and functional groups: catalytic transformation of renewable resources, environmentally benign catalytic addition of small molecules, and development of new materials for high performance devices.
Team Leader, Dr. Masaru Yoshida(D. Sci.)
研究テーマ
- Synthesis of Useful Chemicals from Biomass
- Alcohol Synthesis Using Carbon Dioxide
- New Synthesis of Phosphorescent Materials for Organic Light-Emitting Diodes (OLEDs)
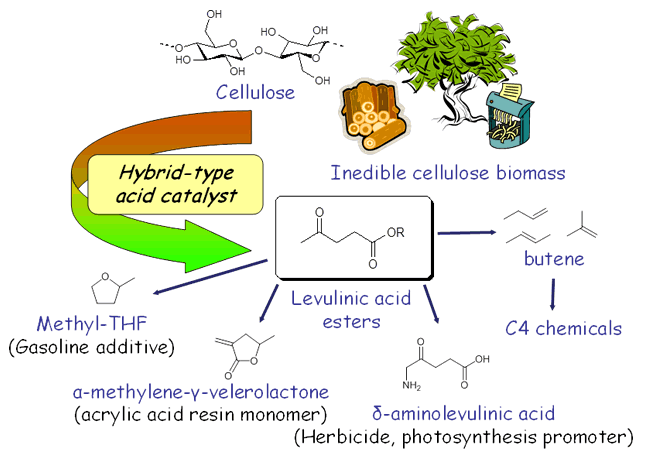
Synthesis of Useful Chemicals from Biomass
We have developed a new catalyst which directly converts cellulose, a main component of plants, into levulinic acid esters in good yields. Levulinic acid is promising as a key building block for fuels, polymers, and fine chemicals.
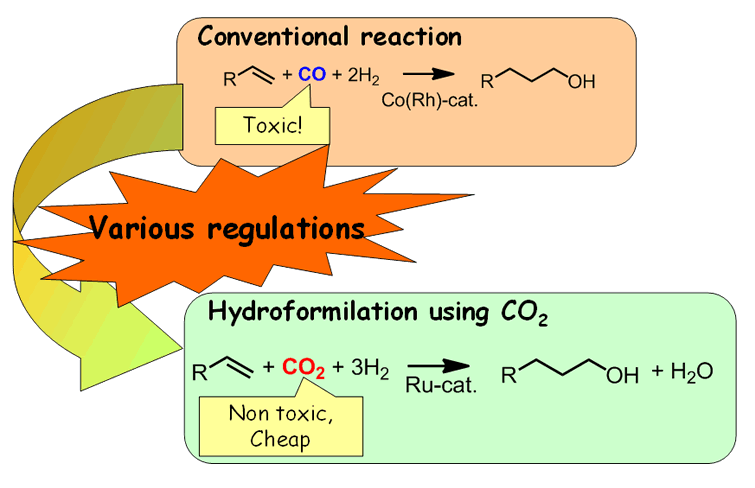
Alcohol Synthesis Using Carbon Dioxide
Hydroformylation (oxo reaction) is one of the key processes in the chemical industry to produce more than 10 million tons of chemicals every year. We have developed new hydro-formylation using CO2 as a reactant in place of toxic CO.
New Synthesis of Phosphorescent Materials for Organic Light-Emitting Diodes (OLEDs)
メンバー
-
Team Leader
Masaru Yoshida (D. Sci.)
masaru.yoshida
-
Chief Senior Researcher
Hajime Kawanami (D. Sci.)
h-kawanami
-
Senior Researcher
Hideo Konno (D. Eng.)
h-konno
-
Senior Researcher
Koji Nemoto (Ph. D.)
k-nemoto
-
Postdoctoral Researcher
Atsushi Yamamoto (D. Sci.)
atsushi.yamamoto
-
Postdoctoral Researcher
Hideaki Ono (ph. D.)
hideaki-ono
*Please add “@aist.go.jp” after each local part.
研究成果
Publication List
| Title | Member | Bibliographic data |
|---|---|---|
| Defining Pt-compressed CO2 synergy for selectivity control of furfural hydrogenation | M. Chatterjee, A. Chatterjee, T. Ishizaka, H. Kawanami | RSC Adv., 8, 20190-20201(2018) |
| Continuous and Sequential Production of High-pressure H2 and high-pressure CO2 from Formic acid | M. Iguchi, M. Chatterjee, N. Onishi, Y. Himeda, H. Kawanami | Sustain. Energy Fuels., 2, 1719-1725(2018) |
| Unlocking compressed CO2 for hydrogenation of concentrated furfural: A fundamental approach to deactivation | M. Chatterjee, A. Chatterjee, T. Ishizaka, I. Nagao, M.i Iguchi, H. Kawanami | Green Chem., 20, 2345-2355(2018) |
| Interconversion between CO2 and HCOOH catalyzed by PdAu nano particle supported by amine functionalized reduced graphene oxide as a dual catalyst | H. Zhong, M. Iguchi, M. Chatterjee, T. Ishizaka, Q. Xu, H. Kawanami | ACS Catalysis., 8, 5355-5362(2018) |
| Formic Acid-based Liquid Organic Hydrogen Carrier System with Heterogeneous Catalysts | H. Zhong, M. Iguchi, M. Chatterjee, Y. Himeda, Q. Xu, H. Kawanami | Adv. Sustainable Syst., DOI:10.1002/adsu.201700161(2018) |
| Development of Proton-Responsive Catalysts | L. Wang, R. Kanega, H. Kawanami, Y. Himeda | Chem Rec., 17, 11, 1071-1094(2017) |
| Effect of the Ortho-Hydroxyl Groups on Bipyridine Ligand of Iridium Complexes for High-Pressure Gas Generation from Catalytic Decomposition of Formic Acid | M. Iguchi, H. Zhong, Y. Himeda, H. Kawanami | Chem. Eur. J., 23, 17788-17793(2017) |
| Kinetic Studies on Formic Acid Dehydrogenation Catalyzed by an Iridium Complex towards Insights into the Catalytic Mechanism of High-Pressure Hydrogen Gas Production | M. Iguchi, H. Zhong, Y. Himeda, H. Kawanami | Chem. Eur. J., 23, 17017-17021(2017) |
| Automatic high-pressure hydrogen generation from formic acid in the presence of nano-Pd heterogeneous catalysts at mild temperatures | H. Zhong, M. Iguchi, F. Song, M. Chatterjee, T. Ishizaka, I. Nagao, Q. Xu, H. Kawanami | Sustain. Energy Fuels., 1, 5, 1049-1055(2017) |
| Development of a Newly Catalytic Plate-type Reactor and Its Evaluation of Methane Conversion and Pressure Drop in Dry Reforming of Methane | T. Fukuda, H. Kawanami, A. Miyazawa | J. Chem. Eng. Japan., 50, 8, 657-665(2017) |
| Aqueous phase homogeneous formic acid disproportionation into methanol | K. Sordakis, A. Tsurusaki, M. Iguch, H. Kawanami, Y. Himeda, G. Laurenczy | Green Chem., 19, 10, 2371-2378(2017) |
| Dehydrogenation of 5-hydroxymethylfurfural to diformylfuran in compressed carbon dioxide: an oxidant free approach | M. Chatterjee, T. Ishizaka, A. Chatterjee, H. Kawanami | Green Chem., 19, 5, 1315-1326(2017) |
| Hydrogenolysis/hydrogenation of diphenyl ether as a model decomposition reaction of lignin from biomass in pressurized CO2/water condition | M. Chatterjee, T. Ishizaka, H. Kawanami | Catal. Today., 281, 402-409(2017) |
| Dehydrogenation of Formic Acid Catalyzed by a Ruthenium Complex with an N,N ‘-Diimine Ligand | C. Guan, D. Zhang, Y. Pan, M. Iguchi, M. J. Ajitha, J. Hu, H. Li, C. Yao, M. Huang, S. Min, J. Zheng, Y. Himeda, H. Kawanami, K. Huang | Inorg. Chem., 56, 1, 438-445(2017) |
| Iridium Catalysts with Diazole-containing Ligands for Hydrogen Generation by Formic Acid Dehydrogenation | Y. Manaka, N. Onishi, M. Iguchi, H. Kawanami, Y. Himeda | J. Japan Pet. Inst., 60, 1, 53-62(2017) |
| TQOPEN (N,N,N’,N’-Tetrakis(2-quinolylmethyl)-3-oxa-1,5-pentanediamine) Family as Heptadentate Fluorescent Cd2+ Sensors | Y. Mikata, A. Kizu, K. Nozaki, H. Konno, H. Ono, S. Mizutani, S. Sato | Inorg. Chem., 56, 7404-7415(2017) |
| A Reactor System Using Electrospray in the Liquid Phase and Its Application in Selective Cyclosiloxane Synthesis | Y. Ido, H. Kobara, K. Tominaga, A. Wakisaka | Ind. Eng. Chem. Res., 56(16), 4878-4882(2017) |
| Replacement of quinolines with isoquinolines affords target metal ion switching from Zn2+ to Cd2+ in the fluorescent sensor TQLN(N,N,N’,N’-tetrakis(2-quinolylmethyl)-2,6-bis(aminomethyl)pyridine) | Y. Mikata, A. Takekoshi, M. Kaneda, H. Konno, K. Yasuda, M. Aoyama and S. Tamotsu | Dalton Trans., 46, 632-637 (2017) |
| Carbon Dioxide to Methanol: The Aqueous Catalytic Way at Room Temperature | K. Sordakis, A. Tsurusaki, M. Iguchi,H. Kawanami, Y. Himeda, G. Laurenczy | Chem. Eur. J., 22, 44, 15605-15608(2016) |
| Development of an Iridium-Based Catalyst for High-Pressure Evolution of Hydrogen from Formic Acid | M. Iguchi, Y. Himeda, Y. Manaka, H. Kawanami | ChemSusChem., 9, 19, 2749-2753(2016) |
| Rapid production of benzazole derivatives by a high-pressure and high-temperature water microflow chemical process | I. Nagao, T. Ishizaka, H. Kawanami | Green Chem., 18, 12, 3494-3498(2016) |
| Simple Continuous High-Pressure Hydrogen Production and Separation System from Formic Acid under Mild Temperatures | M. Iguchi, Y. Himeda, Y. Manaka, K. Matsuoka, H. Kawanami | ChemCatChem., 8, 5, 886-890(2016) |
| Reductive amination of furfural to furfurylamine using aqueous ammonia solution and molecular hydrogen: an environmentally friendly approach | M. Chatterjee, T. Ishizaka, H. Kawanami | Green Chem., 18, 2, 487-496(2016) |
| Neuroprotective effect of Picholine virgin olive oil and its hydroxycinnamic acids component against β-amyloid-induced toxicity in SH-SY5Y neurotypic cells. | M. O. Villareal, K. Sasaki, D. Margout, C. Savry, Z. Almaksour, M. Larroque, H. Isoda | Cytotechnology, 68(6), 2567-2578 (2016) |
| Fluorescent Detection of Phosphate lon via a Tetranuclear Zinc Complex Supported by a Tetrakisquinoline Ligand and μ4-PO4 Core | Y. Mikata, R. Ohnishi, R. Nishijima, H. Konno | Inorg. Chem., 55, 11440-11446 (2016) |
| Efficient nickel-catalyzed hydrocyanation of alkenes using acetone cyanohydrin as a safer cyano source | K. Nemoto, T. Nagafuchi, K. Tominaga, K. Sato | Tetrahedron Lett., 57, 3199 (2016) |
| A practical and efficient synthesis of methyl levulinate from cellulosic biomass catalyzed by an aluminum-based mixed acid catalyst system | K. Tominaga, K. Nemoto, Y. Kamimura, A. Yamada, Y. Yamamoto, K. Sato | RSC Adv., 6, 65119 (2016) |
| OFF-ON-OFF Fluorescent Response of N,N,N’,N’-Tetrakis(1-isoquinolylmethyl)-2-hydroxy-1,3-propanediamine (1-isoHTQHPN) toward Zn2+ | Y. Mikata, R. Ohnishi, A. Ugai, H. Konno, Y. Nakata, I. Hamagami, S. Sato | Dalton Trans., 45, 7250, (2016) |
Cooperative In–Sn catalyst system for efficient methyl lactate synthesis from biomass-derived sugars |
K. Nemoto, Y. Hirano, K. Hirata, T. Takahashi, H. Tsuneki, K. Tominaga, K. Sato | App. Cat. B-Environ., 183, 8, (2016) |
| Rhodium-mediated hydrogenolysis/hydrolysis of the aryl ether bond in supercritical carbon dioxide/water: an experimental and theoretical approach | M. Chatterjee, A. Chatterjee, T. Ishizaka, H. Kawanami | Catal. Sci. Technol., 5, 3, 1532-1539(2015) |
| OFF-ON, Ratiometric, and ON-OFF Fluorescent Responses of Thioether-Linked Bisquinolines toward Hg2+ and Fe3+ Ions | Y. Mikata, K. Nakanishi, F. Nakagaki, A. Kizu, H. Konno | Eur. J. Inorg. Chem., 22, 3769, (2015) |
| Tris(8-methoxy-2-quinolylmethyl)amine (8-MeOTQA) as a highly fluorescent Zn2+ probe prepared by convenient C3-symmetric tripodal amine synthesis | Y. Mikata, Y. Nodomi, R. Ohnishi, A. Kizu, H. Konno | Dalton Trans., 44, 8021, (2015) |
| TQPHEN (N,N,N’,N’-tetrakis(2-quinolylmethyl)-1,2-phenylenediamine) derivatives as highly selective fluorescent probes for Cd2+ | Y. Mikata, A. Kizu, H. Konno | Dalton Trans., 44, 104, (2015) |
| Facile and Efficient Transformation of Lignocellulose into Levulinic Acid Using an AlCl3·6H2O/H3PO4 Hybrid Acid Catalyst | K. Nemoto, K. Tominaga and K. Sato | Bull. Chem. Soc. Jpn.,88, 1752, (2015) |
| Preparation and characterization of PdO nanoparticles on trivalent metal (B, Al and Ga) substituted MCM-41: Excellent catalytic activity in supercritical carbon dioxide | M. Chatterjee, T. Ishizaka, H. Kawanami | J. Colloid Interface Sci., 420, 15-26(2014) |
| Selective hydrogenation of 5-hydroxymethylfurfural to 2,5-bis-(hydroxymethyl)furan using Pt/MCM-41 in an aqueous medium: a simple approach | M. Chatterjee, T. Ishizaka, H. Kawanami | Green Chem., 16, 4734-4739(2014) |
| Hydrogenation of 5-hydroxymethylfurfural in supercritical carbon dioxide-water: a tunable approach to dimethylfuran selectivity | M. Chatterjee, T. Ishizaka, H. Kawanami | Green Chem., 16, 3, 1543-1551(2014) |
| Zinc-specific intramolecular excimer formation in TQEN derivatives: fluorescence and zinc binding property of TPEN-based hexadentate ligands | Y. Mikata, S. Takeuchi, E. Higuchi, A. Ochi, H. Konno, K. Yanai, S. Sato | Dalton Trans., 43, 16377, (2014) |
| Isoquinoline-derivatized tris(2-pyridylmethyl)amines as fluorescent zinc sensors with strict Zn2+/Cd2+ selectivity | Y. Mikata, K. Kawata, S. Takeuchi, K. Nakanishi, H. Konno, S. Itami, K. Yasuda, S. Tamotsu and S. C. Burdette |
Dalton Trans., 43, 10751, (2014)
|
| Bis(2-quinolylmethyl)ethylenediaminediacetic acids (BQENDAs), TQEN-EDTA hybrid molecules as fluorescent zinc sensors | Y. Mikata, S. Takeuchi, H. Konno, S. Iwatsuki, S. Akaji, I. Hamagami, M. Aoyama, K. Yasuda, S. Tamotsu and S. C. Burdette | Dalton Trans., 43, 10013, (2014) |
| Straightforward Synthesis of Levulinic Acid Ester from Lignocellulosic Biomass Resources | K. Nemoto, K. Tominaga and K. Sato | Chem. Lett., 43, 1327, (2014) |
| バイオマス資源を活用した有機合成研究 | 根本耕司 | ペトロテック, 37, 499, (2014) |
| 8-TQEN (N, N, N′, N′-tetrakis(8-quinolylmethyl)ethylenediamine) analogs as fluorescent cadmium sensors: strategies to enhance Cd2+-induced fluorescence and Cd2+/Zn2+ selectivity | Y. Mikata, A. Takekoshi, A. Kizu, Y. Nodomi, M. Aoyama, K. Yasuda, S. Tamotsu, H. Konno and S. C. Burdette | RSC Adv., 4, 12849, (2014) |
| Quinoline-attached triazacyclononane(TACN) derivatives as fluorescent zinc sensors | Y. Mikata, Y. Nodomi, A. Kizu and H. Konno | Dalton Trans., 43, 1684, (2014). |
| Photocatalytic reduction of CO2 under supercritical CO2 conditions: Effect of pressure, temperature, and solvent on catalytic efficiency | H. Kawanami, D. C. Grills, T. Ishizaka, M. Chatterjee, A. Suzuki | J. CO2 Util., 3-4, 93-97(2013) |
| Potential-Dependent Restructuring and Hysteresis in the Structural and Electronic Transformations of Pt/C, Au(Core)-Pt(Shell)/C, and Pd(Core)-Pt(Shell)/C Cathode Catalysts in Polymer Electrolyte Fuel Cells Characterized by in Situ X-ray Absorption Fine Structure, | S. Nagamatsu, T. Arai, M. Yamamoto, T. Ohkura, H. Oyanagi, T. Ishizaka, H. Kawanami, T. Uruga, M. Tada, Y. Iwasawa | J. Phys. Chem. C., 117, 25, 13094-13107(2013) |
| An efficient cleavage of the aryl ether C-O bond in supercritical carbon dioxide-water | M. Chatterjee, T. Ishizaka, A. Suzuki, H. Kawanami | Chem Comm., 49, 40, 4567-4569(2013) |
| Rapid Hydrogenation of Aromatic Nitro Compounds in Supercritical Carbon Dioxide: Mechanistic Implications via Experimental and Theoretical Investigations | M. Chatterjee, A. Chatterjee, H. Kawanami, T. Ishizaka, T. M. Suzuki, A. Suzuki | Adv. Synth. Catal., 354, 10, 2009-2018(2013) |
| Reverse Water-Gas Shift Reaction Catalyzed by Mononuclear Ru Complexes | K. Tsuchiya, J. -D. Huang, and K. Tominaga | ACS Catal., 3, 2865, (2013) |
| 触媒変換プロセスによるセルロース系バイオマスからの有用化学品合成 | 富永 健一 | AIST Today, 13, 14, (2013) |
| 触媒を使って植物から有用な化学品を作る | 富永 健一 | JIR常陽産研ニューズ, 278, 7, (2013) |
| Formation of η2-Coordinated Dihydropyridine–Ruthenium(II) Complexes by Hydride Transfer from Ruthenium(II) to Pyridinium Cations | Y. Matsubara, T. Kosaka, K. Koga, A. Nagasawa, A. Kobayashi, H. Konno, C. Creutz, K. Sakamoto, and O. Ishitani | Organometallics, 32, 6162, (2013) |
| 有機EL青色燐光材料の分子設計 | 今野英雄、井戸洋平 | 月刊ディスプレイ, 19, 19 (2013) |
| Quinoline-based fluorescent zinc sensors with enhanced fluorescence intensity, Zn/Cd selectivity and metal binding affinity by conformational restriction | Y. Mikata, Y. Sato, S. Takeuchi, Y. Koroda, H. Konno, S. Iwatsuki | Dalton Trans., 42, 9688 (2013) |
| Production of organic acids from alginate in high temperature water | T. M. Aida, T. Yamagata, C. Abe, H. Kawanami, M. Watanabe, R. L. Smith | J Supercrit Fluids., 65, 39-44(2012) |
| Continuous Fabrication of Novel Polyimide Nanoparticles Confining Highly Dispersed Gold Nanoparticles by a Multistep Microfluidic Reaction System and Their Catalytic Application | T. Ishizaka, A. Ishigaki, M. Chatterjee, A. Suzuki, T. M. Suzuki, H. Kawanami | Chemistry Letters., 41, 3, 447-449(2012) |
| A Facile and Continuous Fabrication of Polyimide Hollow Nanoparticles Using a Microfluidic System | T. Ishizaka, A. Ishigaki, A. Suzuki, T. M. Suzuki, H. Kawanami | Chemistry Letters., 41, 3, 221-223(2012) |
| Dynamic control of gold nanoparticle morphology in a microchannel flow reactor by glucose reduction in aqueous sodium hydroxide solution | T. Ishizaka, A. Ishigaki, H. Kawanami, A. Suzuki, T. M. Suzuki | J. Colloid Interface Sci., 367, 135-138(2012) |
| In situ synthesized Pd nanoparticles supported on B-MCM-41: an efficient catalyst for hydrogenation of nitroaromatics in supercritical carbon dioxide | M. Chatterjee, T. Ishizaka, T. M. Suzuki, A. Suzuki, H. Kawanami | Green Chem., 14, 12, 3415-3422(2012) |
| 有機EL燐光材料の開発 | 今野英雄 | J. Jpn. Soc. Color Mater., 85, 489 (2012) |
| Quinoline-Based, Glucose-Pendant Fluorescent Zinc Probes | Y. Mikata, A. Ukai, K. Yasuda, S. Itami, T. Tamotsu, H. Konno, S. Iwatsuki | Chem. Biodiv., 9, 2064 (2012) |
| 有機EL燐光材料の製造法と開発動向 | 今野英雄 | Mater. Stage, 11, 60 (2012) |
| 有機EL青色燐光材料の開発動向 | 今野英雄 | クリーンテクノロジー, 22, 49 (2012) |
| Zinc-Specific Fluorescent Response of Tris(isoquinolylmethyl)amines (isoTQAs) | Y. Mikata, K. Kawada, S. Iwatsuki, H. Konno | Inorg. Chem., 51, 1859 (2012) |
| Green Synthetic Method and Simple Size-control of Polyimide Nanoparticles in ScCO2 | T. Ishizaka, A. Ishigaki, T. M. Suzuki, A. Suzuki, H. Kawanami | Chemistry Letters., 40, 8, 849-851(2011) |
| Hydrogenation of aniline to cyclohexylamine in supercritical carbon dioxide: significance of phase behaviour | M. Chatterjee, H. Kawanami, T. Ishizaka, M. Sato, T. M. Suzuki | Appl. Catal. A., 396, 186-193(2011) |
| Sonogashira C-C coupling reaction in water using tubular reactors with catalytic metal inner surface | R. Javaid, H. Kawanami, M. Chatterjee, T. Ishizaka, A. Suzuki, T. M. Suzuki | Chem. Eng. J., 167, 2-3, 431-435(2011) |
| Highly selective non-catalytic Claisen rearrangement in a high-pressure and high-temperature water microreaction system | H. Kawanami, M. Sato, M. Chatterjee, N. Otabe, T. Tsuji, Y. Ikushima, T. Ishizaka, T. Yokoyama, T. M. Suzuki | Chem. Eng. J., 394, 209-214(2011) |
| Selective catalytic oxidation of geraniol to citral with molecular oxygen in supercritical carbon dioxide | S. E. Dapurkar, H. Kawanami, M. Chatterjee , C. V. Rode, T. Yokoyama, Y. Ikushima | Appl. Catal. A., 167, 2-3, 572-577(2011) |
| An attempt to achieve the direct hydrogenolysis of tetrahydrofurfuryl alcohol in supercritical carbon dioxide | M. Chatterjee, H. Kawanami, T. Ishizaka, M. Sato, T. M. Suzuki, A. Suzuki | Catal. Sci. Technol., 1, 1466-1471(2011) |
| Microwave-Assisted Synthesis of Metal Complexes | T. Abe, T. Miyazawa, Y. Kawanishi, H. Konno | Mini Rev. Org. Chem., 8, 315 (2011) |
| Mixed-acid systems for catalytic synthesis of methyl levulinate from cellulose | K. Tominaga, A. Mori, Y. Fukushima, S. Shimada, K. Sato | Green Chem., 13, 810 (2011) |
| Methoxyquinoline-diethylenetriamine conjugate as a fluorescent zinc sensor | Y. Mikata, A. Yamashita, K. Kawada, H. Konno, S. Itami, K. Yasuda, T. Tamotsu | Dalton Trans., 40, 4976 (2011) |
| Methoxy-substituted isoTQEN family for enhanced fluorescence response toward zinc ion | Y. Mikata, A. Yamashita, K. Kawada, H. Konno, S. Itami, K. Yasuda, T. Tamotsu | Dalton Trans., 40, 4059 (2011) |
| Development of an Efficient and Durable Photocatalytic System for Hydride Reduction of an NAD(P)+ Model Compound Using a Ruthenium(II) Complex Based on Mechanistic Studies | Y. Matsubara, Y. Koga, A. Kobayashi, H. Konno, K. Sakamoto, I. Morimoto, O. Ishitani | J. Am. Chem. Soc., 132, 10547 (2010) |
| Deuteration isotope effect on nonradiative transition of fac-tris (2-phenylpyridinato) iridium (III) complexes |
T. Abe, T. Miyazawa, H. Konno, Y. Kawanishi | Chem. Phys. Lett., 491, 199 (2010) |
| An environmentally friendly hydroformylation using carbon dioxide as a reactant catalyzed by immobilized Ru-complex in ionic liquids | K. Tominaga | Catal Today, 115, 70 (2006) |
Invited/Requested Iectures
| Subject Title | Society Name | Date | Presenter |
|---|---|---|---|
| ギ酸からの高圧水素製造技術開発 | 日本化学会第100春期年会 | 2020.3 ※開催中止(発表成立) | 川波 肇 |
| High-pressure gas generation by formic acid dehydrogenation | Workshop of “Catalysis & Energy” in Leibniz-Institute für Katalyse e.V. (LIKAT) | 2019.11 | 川波 肇 |
| ギ酸からの高圧水素製造技術 | INCHEM TOKYO 2019 | 2019.11 | 川波 肇 |
| High-pressure Hydrogen Gas Production by Formic Acid Dehydrogenation | 7th Asian Conference on Coordination Chemistry 2019 | 2019.10 | 川波 肇 |
| High-pressure H2 and CO2 production and Separation Process from Formic Acid | World Hydrogen Technologies Convention 2019 | 2019.6 | 川波 肇, 姫田雄一郎 |
| High-pressure hydrogen production over 150 MPa from formic acid dehydrogenation catalyzed by homogeneous Ir catalysts | 3rd International Conference on Catalysis and Chemical Engineering | 2019.2 | 川波 肇 |
| Catalytic Reaction in High-pressure and High-temperature Water Including Supercritical Water | International Conference on Coordination Chemistry 2018 | 2018.8 | 川波 肇 |
| High-pressure Hydrogen Production by Homogeneous Catalysts and H2/CO2 Separation System from Formic Acid | XXVII International Conference on Organometallic Chemistry | 2018.7 | 川波 肇, 井口 昌幸, A. Chatterjee, 西 尚, 姫田 雄一郎 |
| High-pressure Hydrogen Production by Homogeneous Catalysts and H2/CO2 Separation System from Formic Acid | XXVII International Conference on Organometallic Chemistry | 2018.7 | 川波 肇, 井口 昌幸, A. Chatterjee, 西 尚, 姫田 雄一郎 |
| ギ酸の脱水素化反応による水素生成のためのジアゾールを配位子に含むイリジウム触媒 | 石油学会第61年会 | 2018.5 | 眞中雄一, 尾西直弥, 井口昌幸, 川波 肇, 姫田 雄一郎 |
| Development of continuous high-pressure hydrogen evolution from formic acid in the presence of nano-Pd heterogeneous catalysts | 255th ACS National Meeting | 2018.3 | 川波 肇, 鍾 恒, 井口 昌幸, 石坂 孝之, 徐 強 |
| 反応と分離の連続操作によるギ酸からの水素製造と二酸化炭素の回収 | 化学工学会第83年会 | 2018.3 | 井口 昌幸, 姫田 雄一郎, 川波 肇 |
| 超臨界流体を利用した有機合成 | 超臨界流体基礎セミナー | 2018.1 | 川波 肇 |
| 高温高圧下における各種有機反応(水と二酸化炭素を例に) | 熊本大学大学院先端化学研究部講演会 | 2017.12 | 川波 肇, C. May, 石坂 孝, 長尾 育 |
| Hydrogenation and dehydrogenation of 5-hydroxymethylfurfural to diformylfuran in compressed carbon dioxide | World Chemistry Conference and Exhibition | 2017.9 | 川波 肇 |
| High-pressure hydrogen production from formic acid in the presence of homogeneous catalyst and heterogeneous catalyst | ICH2P (International Conference on Hydrogen Production) | 2017.7 | 川波 肇, 井口 昌, 鍾 , 姫田 雄一, 徐 強 |
| Automatic high-pressure hydrogen generation from formic acid in the presence of nano-Pd heterogeneous catalyst | The 1st SJTU Environmental International Forum for Young Scholars | 2017.5 | 鍾 恒,川波 肇 |
| High-pressure hydrogen and Carbon Dioxide from Formic Acid without any compressing procedure | 3rd International Conference on Molecular and Functional Catalysis | 2017.2 | 川波 肇 |
| 高温高圧水・超臨界水を用いた有機合成 | 第一工業製薬(株)研究発表大会 特別講演会 | 2017.1 | 川波 肇 |
| 超臨界流体を利用した有機合成 | 超臨界流体基礎セミナー | 2017.1 | 川波 肇 |
| High-pressure Hydrogen Evolution from Formic Acid | 2017 International Workshop for Collaboration in Hydrogen Energy | 2017.1 | 川波 肇 |
| The exploration research into medicinal functions of Aurantiochytrium and Botryococcus braunii using bioassay | The 10th annual Algae Biomass Summit | 2016.10 | Hiroko Isoda,・K. Sasaki・M. Sakamaki・S. Takahashi・M. Watanabe・M. Demura・M. Yoshida・H. Kigoshi・M. Kita・K. Tominaga |
| High-pressure chemistry with H2 & CO2 | Innovation for Cool Earth Forum | 2016.10 | 川波 肇 |
| High-pressure gas evolution from formic acid catalyzed Ir catalyst and continuous separation of H2/CO2 | The 3rd European Colloquium on Inorganic Reaction Mechanism 2016 | 2016.6 | 川波 肇, 井口 昌幸, 姫田 雄一郎 |
| 高圧技術を駆使した各種化学プロセスの構築 | 大分大学工学部講演会 | 2016.4 | 川波 肇 |
| 高温高圧マイクロリアクターを用いた物質製造プロセス技術 | (株)情報機構セミナー | 2016.3 | 川波 肇 |
| 水素価格を半減できる、注目の産総研『高圧水素供給技術』 | みんなの環境エネルギー会議/エネルギーイノベーション研究会 | 2016.1 | 川波 肇 |
| Continuous High Pressure Hydrogen Evolution System with Formic Acid without Compressing | International Chemical Congress of Pacific Basin Societies 2015 | 2015.12 | 川波 肇, 井口 昌幸, 眞中 雄一, 姫田 雄一郎 |
| Hydrogen Production by Dehydrogenation of Formic Acid using Homogeneous Iridium Catalysts | Pacifichem | 2015.12 | 姫田 雄一郎, 藤田恵津子, J. T. Muckerman, 川波 肇, 井口 昌幸, 眞中 雄一, 姫田 雄一郎 |
| Continuous effective conversion of woody chips to the HMF derivatives by using high-pressure and high-temperature water | France-Canada-Japan Workshop 2015 | 2015.7 | 川波 肇, 姫田 雄一郎 |
| Organic synthesis using supercritical and subcritical water | 産総研ナノ機能合成グループ講演会 | 2015.3 | 川波 肇 |
| 高温高圧マイクロリアクターを用いた各種物質製造技術の開発 | JRS講演会 | 2015.2 | 川波 肇 |
| 高温高圧とマイクロリアクターの組み合わせによる有機材料合成への展開 | フローマイクロ合成研究会第64回研究会 | 2014.10 | 川波 肇 |
| 高温高圧水ーマイクロ反応場による各種有機反応技術 | 化学工学会 超臨界流体部会 第13回 サマースクール | 2014.8 | 川波 肇 |
| 光反応における高圧流体の効果 | 産総研シンポジウム | 2014.2 | 川波 肇 |
| Organic synthesis using supercritical and subcritical water | Mini workshop on Supercritical Fluid | 2013.10 | 川波 肇 |
| Efficient Alcohol Synthesis by Hydrogenation of Ether Bond in The Presence of Heterogeneous Catalyst in Supercritical Carbon Dioxide | 246th ACS National Meeting and Exposition | 2013.9 | 川波 肇, C. Maya, 石坂 孝之 |
| 循環型原料を用いた有用化学品合成のための触媒開発 | 第34回触媒学会若手会「夏の研修会」 | 2013.7 | 富永健一 |
| Substitutent Effects of ReCl(bpy)(CO)3 Catalyst for the Photocatalytic Reduction of CO2 to CO in scCO2 | BNL Inorganic Group Meeting | 2013.6 | 川波 肇 |
| 高温高圧を用いた各種物質製造技術の開発~二酸化炭素と水~ | 日本レオロジー学会高分子加工技術研究会 | 2013.6 | 川波 肇 |
| バイオマス資源を用いた有用化学品合成のための有機合成戦略 | 理研バイオマス若手研究会 | 2013.6 | 根本耕司 |
| 有機ELリン光材料の開発 | 三菱ガス化学株式会社講演会 | 2012.9 | 今野英雄 |
| 高温高圧水・超臨界水を用いた有機合成にむけた取り組み | 化学工学会反応工学部会若手会(反好会)講演会 | 2012.9 | 川波 肇, C. Maya, 石坂 孝之 |
| レブリン酸を基幹物質とするバイオマスリファイナリー | 触媒資源化協会講演会 | 2012.7 | 富永健一 |
| 有機EL素子と有機材料~りん光材料の製法開発~ | 触媒資源化協会講演会 | 2012.7 | 今野英雄 |
| レブリン酸を基幹物質とするバイオマスリファイナリー | 三菱ガス化学株式会社講演会 | 2012.7 | 富永健一 |
| 高温高圧条件下におけるマイクロリアクターを用いた有機合成法 | 日本ポリプラスチックス株式会社講演会 | 2012.5 | 川波 肇 |
| 高温高圧マイクロリアクターを用いた物質製造プロセス技術の開発 | 医薬品原料・中間体展(CPhI)プロセス化学セミナー | 2012.3 | 川波 肇 |
| 高温高圧プロセスを駆使した環境にやさしい超高速有機合成技術 | 医薬品原料・中間体展(CPhI)技術移転セミナー | 2012.3 | 川波 肇 |
| Development of a Chemical Process for the Photoreduction of CO2 Under High-Pressure Conditions | Japan-U.S. Collaborationon Clean Energy Technology Workshop 2012 | 2012.2 | 川波 肇 |
| 有機EL燐光材料の製造方法と開発動向 | 株式会社技術情報協会セミナー | 2011.12 | 今野英雄 |
| 高温高圧水—マイクロリアクター物質合成技術 | INCHEMTOKYO | 2011.11 | 川波 肇 |
| 耐高温高圧マイクロリアクターを用いた高効率物質製造法 | 化学工学会第43回秋季大会 | 2011.9 | 川波 肇 |