TEAMDigital-Driven Chemistry Team

The Flow and Digital-Driven Chemistry Team is a newly formed team in April 2024 through the merger of the Flow Chemistry Team and the Digital-Driven Chemistry Team. Our team is promoting research and development in line with one of the key issues of the Catalysis Chemistry Research Center: "Continuous Precision Production Process Technology and Data-Driven Reaction Development."
First, we are focusing on the research and development related to the NEDO project "Development of Continuous Precision Production Process Technology for Functional Chemicals," which started in 2019. We are working on developing continuous production technology for functional chemicals. This includes:
Developing reactions and catalysts to convert conventional batch organic reactions into continuous flow methods
Developing reactor modules suitable for continuous flow systems using catalysts
Developing technologies for the continuous and automated downstream processes in organic synthesis (extraction, washing, concentration, crystallization, filtration, drying, etc.)
Furthermore, we are promoting "Digital-Driven Chemistry" research, aiming to integrate chemistry with data science, including artificial intelligence (AI), computational chemistry, automation, and robotics. For example, we are engaged in research and development related to:
Designing functional chemicals (Materials Informatics (MI))
Catalyst design technology (Catalyst Informatics (CI))
Synthetic route design technology (Process Informatics (PI))
In this way, we aim for technological innovation in the chemical industry through the continuous production of functional chemicals. At the same time, we are promoting basic research and practical application research on design and development of new materials, new reactions, and new catalysts, process and plant design, and automatic control.
These efforts utilize digital technologies such as material and process data of substances, chemical reactions, and processes, as well as data science, computational science, and simulators.
Through these activities, we contribute to the digital transformation (DX) of domestic academia and the chemical industry.
Team Leader, Dr. Akira Yada (Ph. D.)
研究テーマ
- Development of digital-driven technology for designing new functional materials and catalysts
- Development of functional soft materials and PI technology by signal processing
- Laboratory automation technology toward new reactions and catalysts
Development of digital-driven technology for designing new functional materials and catalysts
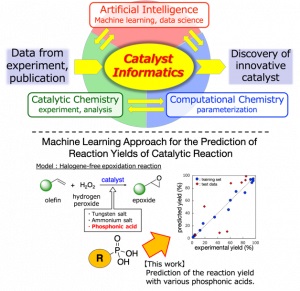
Aiming at the discovery and development of innovative catalysts autonomously, we are working on “catalyst informatics” research that integrates catalytic chemistry, computational chemistry, and data science. We are also developing new technology for designing synthetic and degradation pathways for functional chemicals, which contribute to a carbon-neutral or circular economy that should be accomplished urgently.
References
Chem. Lett. 2018, 43, 283-287 (link, Press release)
Synlett, 2021, 32, 1843-1848 (link)
Development of functional soft materials and PI technology by signal processing
We are developing soft actuators and soft robots by synthesizing functional soft materials and utilizing their functions, and developing capillary gel electrophoresis equipment using gel mesh as a separation medium. We are also conducting research and development on process informatics technology (anomaly detection and predictive maintenance) using machine learning over signal processing.
Laboratory automation technology toward new reactions and catalysts
We aim to automate various unit operations of synthetic chemistry, for overcoming bottlenecks in research and development, to accelerate the discovery of new reactions and the development of highly efficient catalysts. We are promoting research focusing on flow reactions, which have high needs for automation because various unit operations are linked together.
メンバー
-
Team Leader
Akira Yada
a-yada
-
Senior Researcher
Yusuke Hara
y-hara
-
Senior Researcher
Koichiro Masuda
koichiro-masuda
-
Senior Researcher
Kanako Kumada
k.kumada
-
Technical Staff
Kaori Nagaoka
-
Technical Staff
Yukiko Okamoto
-
Temporary Technical staff
Ei-ichi Nakai
-
Temporary Technical staff
Maki Inose
-
Temporary Technical staff
Tadafumi Uchimaru
-
Temporary Technical staff
Naoko Suzuki
-
Visiting Researcher
Masahito Hayashi
* add “@aist.go.jp”
研究成果
-
<2024>
“Gold-Catalyzed Synthesis of Ortho-Quinone Methide Analogues as Reactive Synthetic Precursors”
Yamada, T.*; Fujii, A.; Furugen, C.; Kobayashi, K.; Hyodo, T.; Ikawa, T.; Sajiki, H.*
Adv. Synth. Catal. 2024, 366, 2270.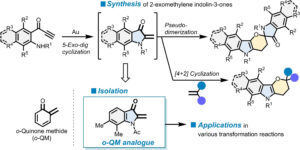
“Continuous synthesis of homoallylic ketones via ketal-Claisen rearrangement using solid-acid catalysts”
Kobahyashi, K.*; Negoro, C.; Takaishi, J.; Masuda, K.*; Kobayashi, S.*
Org. Chem. Front. 2024, 11, 1990.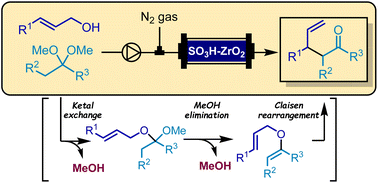
“Enhancing continuous-flow reactions via compression-molding of solid catalysts and dilutants in packed-bed systems”
Kobayashi, K.; Tanaka, T.; Kon, Y.; Kawanami, H.*; Koumura, N.*
RSC Adv. 2024, 14, 6598.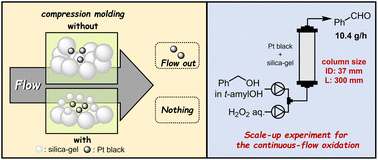
“Copper-Catalyzed Aerobic Benzylic C(sp3)−H Oxidation of Unprotected Aniline Derivatives for the Synthesis of Phenanthridines”
Nozawa-Kumada, K.*; Matsuzawa, Y.; Hayashi, M.; Kobayashi, T.; Shigeno, M.; Yada, A.; Kondo, Y.
Adv. Synth. Catal. 2024, 366, 2241.
“Complete sequence randomness of lactate-based copolymers, LAHBs with varied lactate monomer fractions employing a series of propionyl-CoA transferases”
Koh, S.; Endo, R.; Kahar, P.; Mori, Y.; Ogino, C.; Tanaka, S.; Tanaka, S.; Imai, Y.; Taguchi, S.
Int. J. Biol. Macromol. 2024, 274, 133055.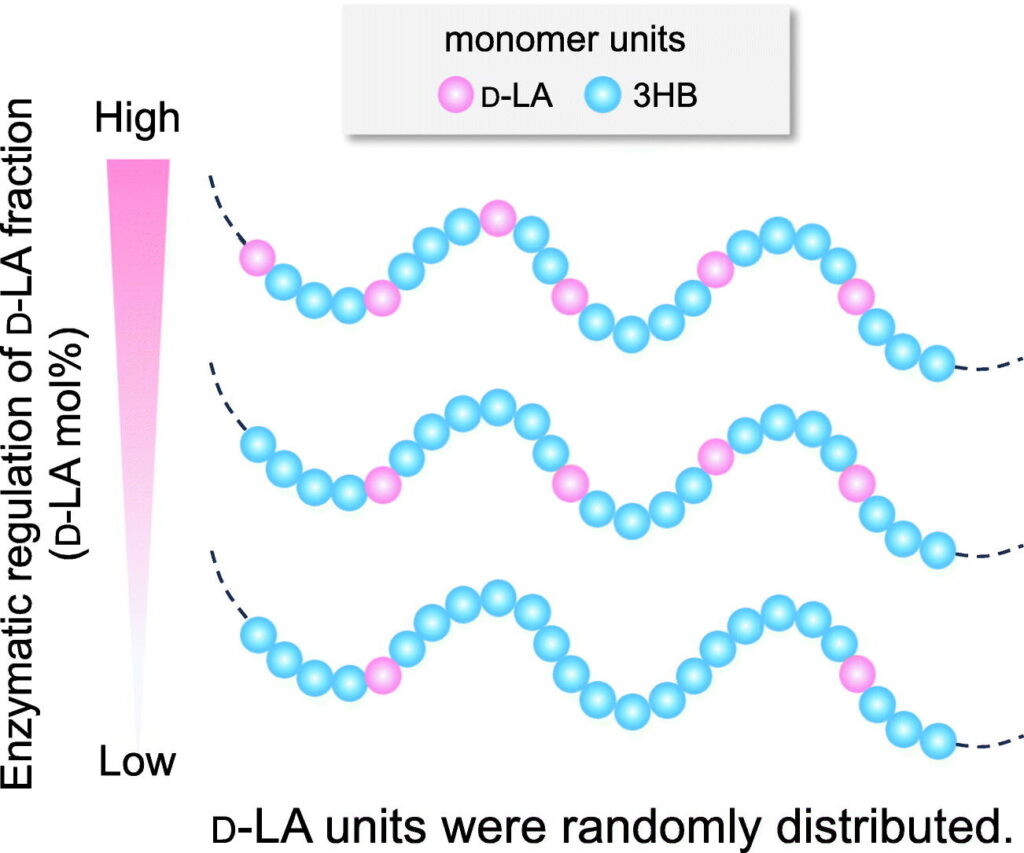
<2023>
“Copper-Catalyzed Intramolecular Olefinic C(sp2)–H Amidation for the Synthesis of γ-Alkylidene-γ-lactams”
Nozawa-Kumada, K.*; Hayashi, M.; Kwon, E.; Shigeno, M.; Yada, A.; Kondo, Y.
Molecules 2023, 28, 6682.
“Machine Learning that Proposes Reaction Conditions and Yields for Wittig-type Methylenation of Aldehydes with Bis(iodozincio)methane in a Flow-microreactor”
Maruoka, T.; Yada, A.;Sato, K.; Matsubara, S.*
Chem. Lett. 2023, 52, 397.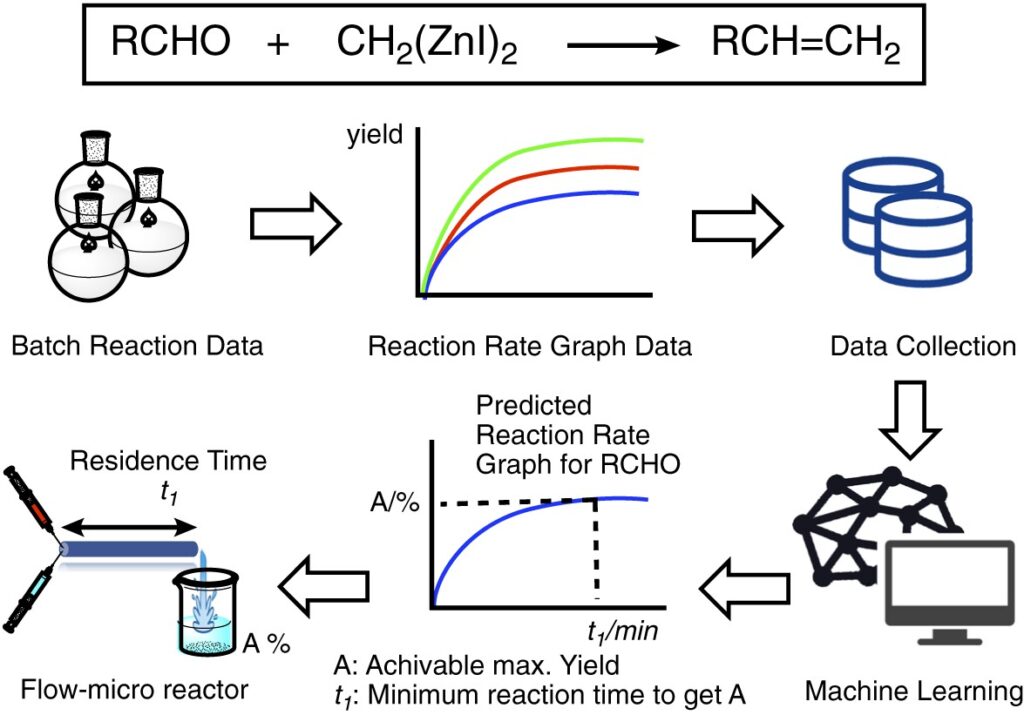
“Machine-Learning Classification for the Prediction of Catalytic Activity of Organic Photosensitizers in the Nickel(II)-Salt-Induced Synthesis of Phenols”
Noto, N.*; Yada, A.; Yanai, T.; Saito, S.*
Angew. Chem. Int. Ed. 2023, 62, e202219107.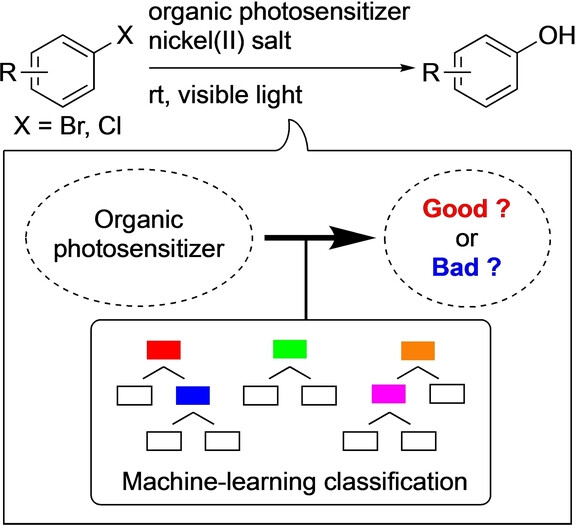
“Aluminium-catalysed synthesis of aryl enol ethers from phenols and dimethyl ketals”
Kobayashi, K.; Komatsuzaki, S.; Onozawa, S.; Masuda, K.; Kobayashi, S.
Org. Biomol. Chem. 2023, 21, 8259-8262.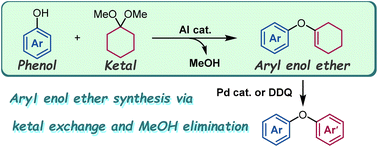

“A Continuous-Flow Method for the Transformation from Amides to Nitriles Catalyzed by CeO2 in Acetonitrile”
Kobayashi, K.; Masuda, K.; Feng, F.; Rashed, Md. N.; Sato, K.; Koumura, N.; Kobayashi, S.
Adv. Synth. Catal. 2023, 365, (10), 1618-1622.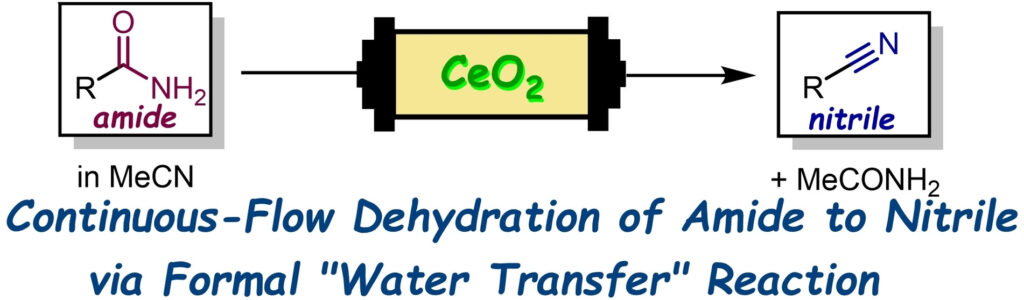
<2022>
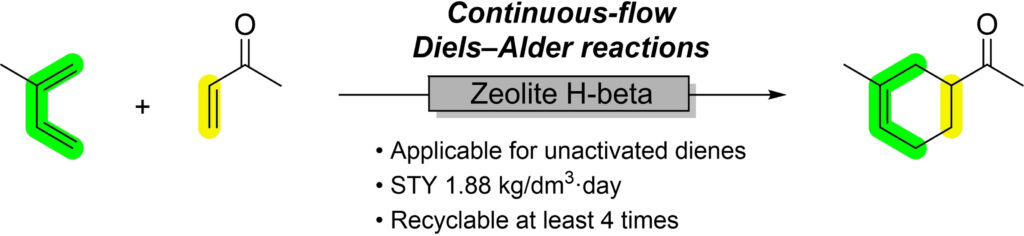
“Continuous-Flow Diels-Alder Reactions of Unactivated Dienes over Zeolitic Catalysts”
Masuda, K.; Agalave, S. G.; Chen, W.; Onozawa, S.; Shimada, S.; Sato, K.; Kobayashi, S.
Asian J. Org. Chem. 2022, 12, e202200382.
“Rapid and Sensitive Determination of Leached Platinum Group Elements in Organic Reaction Solution of Metal-Catalyzed Reactions by Laser Ablation-ICP-MS with Spot-Drying on Paper”
Makino, Y.*; Matsuo, H.; Masuda, K.; Onozawa, S.; Nakazato, T.
J. Anal. At. Spectrom. 2022, 37, 1787.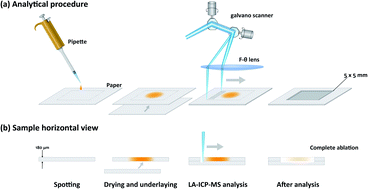
“Efficient Recycling of Catalyst-Solvent Couples from Lewis Acid-Catalyzed Asymmetric Reactions in Water”
Kitanosono, T.*; Lu, F.; Masuda, K.; Yamashita, Y.; Kobayashi, S.*
Angew. Chemie Int. Ed. 2022, 61, e202202335.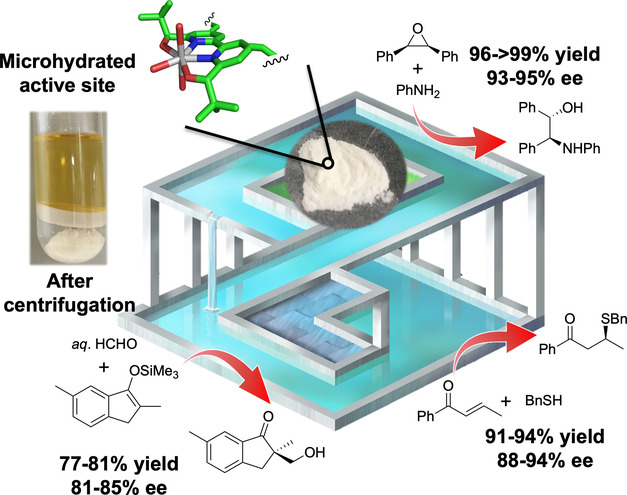
“Synthesis, structure and properties of trivalent and pentavalent tricarbabismatranes”
Shimada, S*; Yin, SF; Choe, YK.
Chem. Commun., 2022, 58, 6614-6617.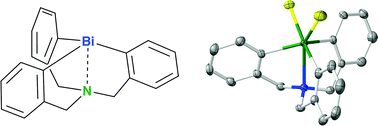
“Oxidative Addition of Water to Ir(I) Complexes Bearing a Pincer-Type Silyl Ligand”
Fang, HY; Shimada, S*
ACS Omega, 2022, 7, 20237-20240.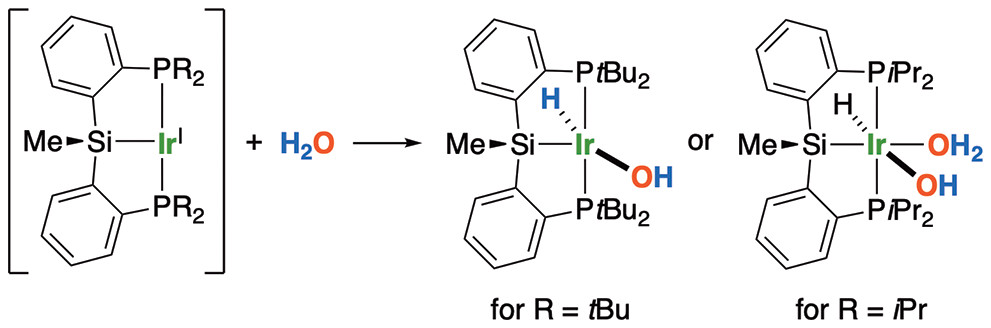
<before 2021>
“Ensemble Learning Approach with LASSO for Predicting Catalytic Reaction Rates”
Yada, A.; Matsumura, T.; Ando, Y.; Nagata , K.; Ichinoseki, S.; Sato, K.
Synlett, 2021, 32, 1843–1848.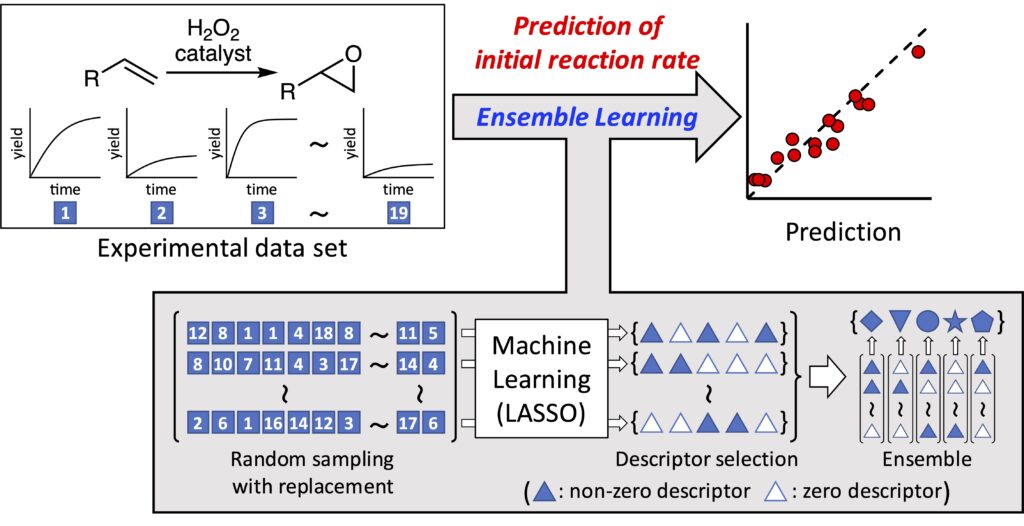
“Aerobic Dehydrogenative Coupling of Naphthols and Phenols with a Ru(OH)x/Al2O3 Catalyst under Continuous-Flow Conditions”
Masuda, K.; Chen, W.; Hayashi, K.; Shimada, S.; Onozawa, S.; Koumura, N.; Sato, K.; Kobayashi, S.
ChemistrySelect, 2021, 6, 10106–10110.
“Development of highly efficient Friedel–Crafts alkylations with alcohols using heterogeneous catalysts under continuous-flow conditions”
Masuda, K.; Okamoto, Y.; Onozawa, S.; Koumura, N.; Kobayashi, S.
RSC Adv. 2021, 11, 24424-24428.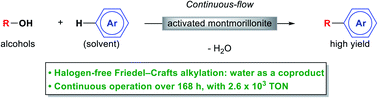
“Stereoretentive N-Arylation of Amino Acid Esters with Cyclohexanones Utilizing a Continuous-Flow System”
Ichitsuka, T.; Komatsuzaki, S.; Masuda, K.; Koumura, N; Sato, K; Kobayashi, S.
Chem. Eur. J. Early View, 10844-10848.
Highlighted in Synfacts and OPRD.
“Zirconium Oxide‐Catalyzed Direct Amidation of Unactivated Esters under Continuous‐Flow Conditions”
Rashed, Md. N.; Masuda, K.; Ichitsuka, T.; Koumura, N.; Sato, K.; Kobayashi, S.
Adv. Synth. Catal. 2021, 363, 2529-2535.
Selected as Very Important Publication (VIP)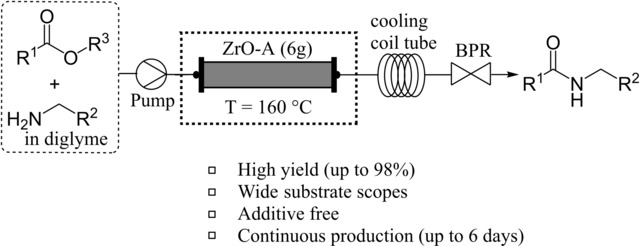

“Continuous Synthesis of Aryl Amines from Phenols Utilizing Integrated Packed‐Bed Flow Systems”Ichitsuka, T.; Takahashi, I.; Koumura, N.; Sato, K.; Kobayashi, S.
Angew. Chem. Int. Ed. 2020, 59, 15891-15896.
Press release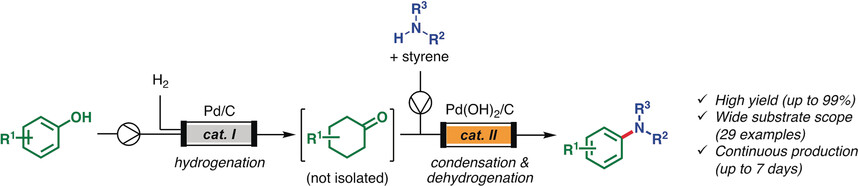
“Robust Organic Photosensitizers Immobilized on a Vinylimidazolium Functionalized Support for Singlet Oxygen Generation under Continuous-Flow Conditions”
Masuda, K.; Wang, Y.; Onozawa, S.; Shimada, S.; Koumura, N.; Sato, K.; Kobayashi, S.
Synlett 2020, 31, 497-501.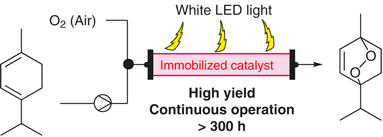
“Readily Available Immobilized Pd Catalysts for Suzuki‐Miyaura Coupling under Continuous‐flow Conditions”
Ichitsuka, T.; Suzuki, N.; Sairenji, M.; Koumura, N.; Onozawa, S.; Sato, K.; Kobayashi, S.
ChemCatChem 2019, 11, 2427-2431.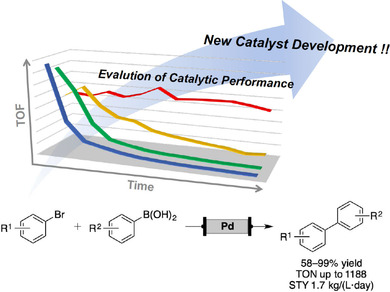
“Novel synthesis of 1,4-thiazin-2-one O-(tert-butyl) oximes and benzo[b][1,4]thiazin-2-one O-(tert-butyl) oximes in the presence of K2CO3/SiO2”
Aoyama, T.; Tashiro, K.; Hayakawa, M.; Shimada, S.; Ouchi, A.
Tetrahedron Lett. 2019, 60, 1493-1497.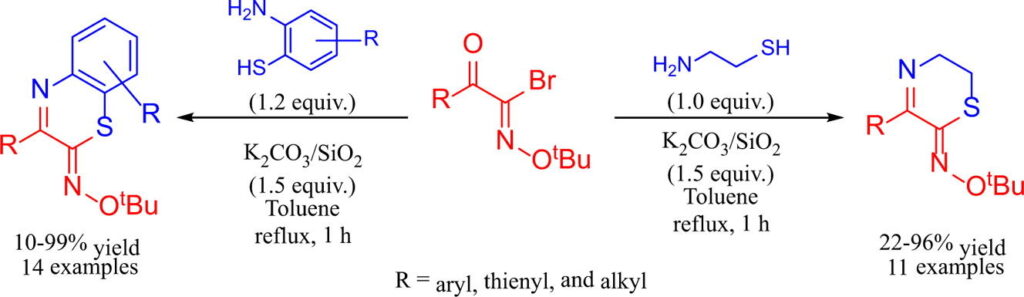
“Machine Learning Approach for Prediction of Reaction Yield with Simulated Catalyst Parameters”
Yada, A.; Nagata, K.; Ando, Y.; Matsumura, T.; Ichinoseki, S.; Sato, K.
Chem. Lett. 2018, 47, 284-287.
Press release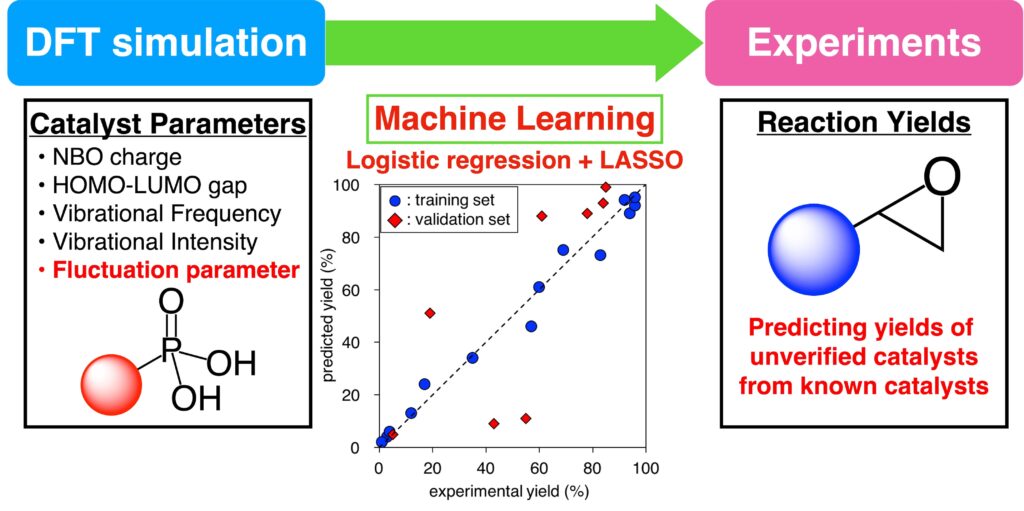
“Regio- and stereoselective addition of trans-Pt(TeAr)(SiMe3)(PEt3)2 to terminal alkynes leading to cis-[(Z)-β-trimethylsilylalkenyl]platinum(II) complexes”
Han, L.-B.; Mirzaei, F.; Shimada, S.
Heteroatom Chem. 2018, 29, e21463.“A simple and efficient method for the synthesis of 5,6-dihydropyrazin-2(1H)-one O-(tert-butyl)oximes, quinoxalin-2(1H)-one O-(tert-butyl)oximes and its derivatives”
Aoyama, T.; Tashiro, K.; Hayakawa, M.; Shimada, S.; Ouchi, A.
Tetrahedron Lett., 2018, 59, 4116-4119.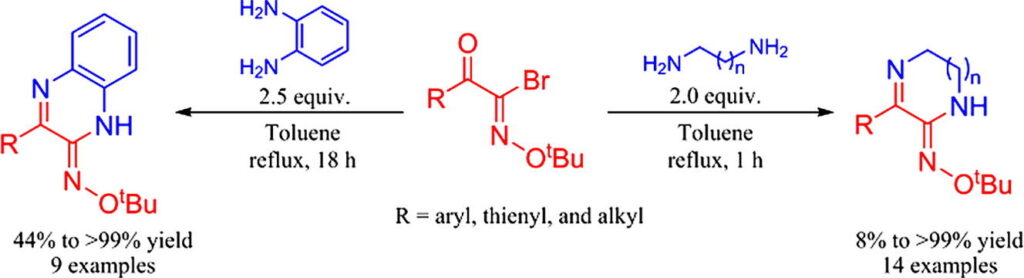
“Iridium-Catalyzed Hydrosilylation of Sulfur-containing Olefins”
Srinivas, V.; Nakajima, Y.; Sato, K.; Shimada, S.
Org. Lett., 2018, 20, 12-15.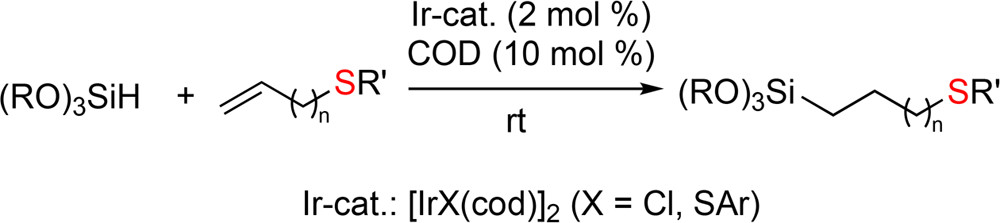
“Flow Fine Synthesis with Heterogeneous Catalysts”
Masuda, K.; Ichitsuka, T.; Koumura, N.; Sato, K.; Kobayashi, S.*
Tetrahedron, 2018, 74, 1705-1730.
- ・Press releases
To be updated
- ・Reviews, books
To be updated